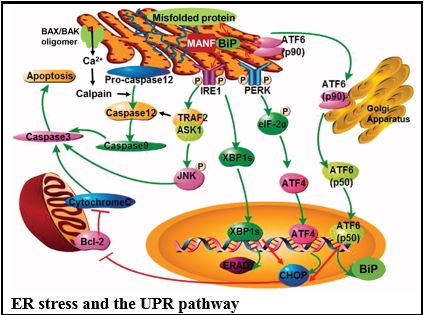
The endoplasmic reticulum (ER) is the central site for folding, post-translational modifications, and transport of secretory and membrane proteins. Environmental and genetic factors disrupting ER function lead to accumulation of misfolded and unfolded proteins in the ER lumen, termed ER stress.
The adaptive response to ER stress is the unfolded protein response (UPR), which is initiated by three ER transmembrane proteins, inositol-requiring enzyme-1 (IRE1), PKR-like ER kinase (PERK), and activating transcription factor 6 (ATF6). Cells rendered dysfunctional due to severe or prolonged ER stress are eliminated from the organism by the proapoptotic UPR, mediated by CHOP, JNK, or caspase 12. The dysregulation of the UPR pathway can result in various diseases such as diabetes, inflammation, and neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease, which are collectively known as “ER diseases”. Accumulating evidence has also highlighted the importance of ER dysfunction in the pathogenesis of various glomerular and tubular diseases.
Our major focuses are primary nephrotic syndrome, Alport syndrome, and autosomal dominant tubulointerstitial kidney disease (ADTKD) caused by podocyte gene mutations, collagen IV mutations, and uromodulin mutations, respectively. In addition to genetic kidney diseases inducing ER stress, we are also interested in acquired forms of ER stress-mediated kidney disease, including ischemic acute kidney injury and renal fibrosis.
We are using a combination of stably transfected cell lines, transgenic, knock-out and knock-in animal models, as well as human biospecimens including DNA, blood, urine and induced pluripotent stem cells, to investigate ER stress-mediated kidney disease. We have assembled an interdisciplinary team including multiple collaborators and core facilities with the required expertise in pioneering ER stress research, human and mouse genetics, next generation sequencing, urine biomarker validation, and high throughput drug screening. We have also acquired access to clinical resources of multiple disease cohorts. The integrated approach will enable us to delineate ER stress signaling cascades, to stratify disease risk, and to develop innovative and highly-targeted ER stress modulators. Our ultimate goal is to design personalized clinical trials to treat kidney disease patients mediated by ER stress in the era of Precision Nephrology.